February 2003 – The CenterWatch Monthly : PDF
Product Details
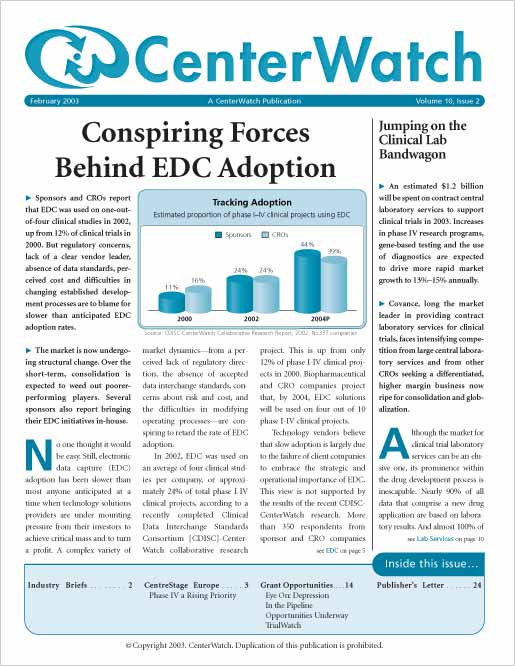
Conspiring Forces Behind EDC Adoption
Sponsors and CROs report that electronic data capture (EDC) was used on one-out-of-four clinical studies in 2002, up from only 12% of clinical trials in 2000. But regulatory concerns, lack of a clear market leader, absence of data standards, perceived cost, and difficulties in changing long-established development processes are to blame for slower than anticipated EDC adoption rates. The market is now undergoing structural change. Over the short-term, consolidation is expected to weed out poorer-performing players. Several sponsors also report bringing their EDC initiatives in-house.
Jumping on the Clinical Trial Lab Services Bandwagon
An estimated $1.2 billion will be spent on contract central laboratory services to support clinical trials in 2003. Increases in phase IV research programs, gene-based testing and the use of diagnostics are expected to drive more rapid market growth to 13%–15% annually. Covance, long the market leader in providing contract laboratory services for clinical trials, faces intensifying competition from large central laboratory services and from other CROs seeking a differentiated, higher margin business now ripe for consolidation and globalization.
Also in this issue:
- CentreStage Europe: Phase IV a Rising Priority
- Eye On: Depression