April 2003 – The CenterWatch Monthly : PDF
$79.00
Product Details
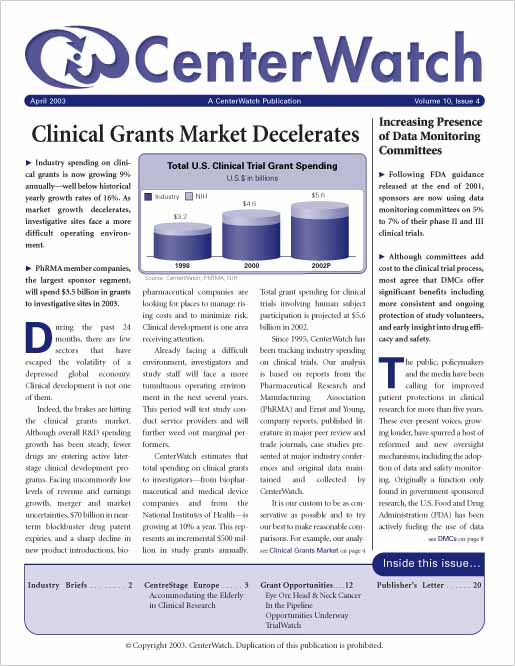
Clinical Grants Market Decelerates
Industry spending on clinical grants is now growing 9% annually–well below historical yearly growth rates of 16%. PhRMA member companies, the largest sponsor segment, will spend $3.5 billion in grants to investigative sites in 2003. As market growth decelerates, investigative sites must brace for a more difficult operating environment.
Increasing Presence of Data Monitoring Committees
Following FDA guidance released at the end of 2001, sponsors are now using data monitoring committees (DMCs) on 5% to 7% of their phase II and III clinical trials. Although committees add cost to the clinical trial process, most agree that DMCs offer significant benefits including more consistent and ongoing protection of study volunteers, and early insight into drug efficacy and safety.
Also in this issue:
- CentreStage Europe: Accommodating the Elderly in Clinical Research
- Eye on: Head and Neck Cancer