April 2019 – The CenterWatch Monthly : PDF
$79.00
Product Details
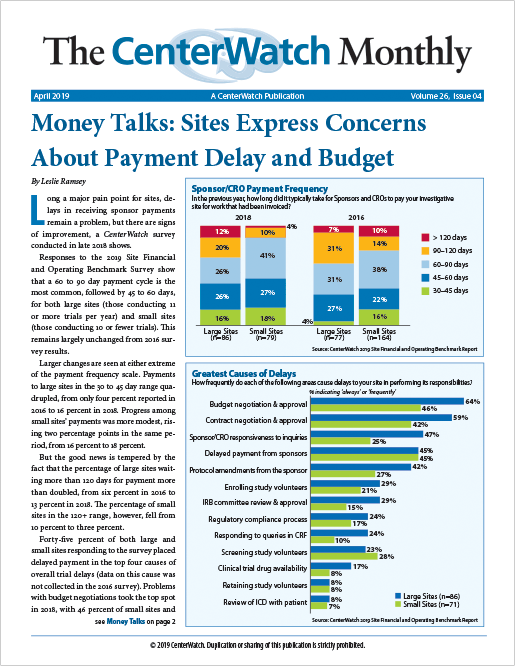
Money Talks: Sites Express Concerns About Payment Delay and Budget
Long a major pain point for sites, delays in receiving sponsor payments remain a problem, but there are signs of improvement, a CenterWatch survey conducted in late 2018 shows.
Responses to the 2019 Site Financial and Operating Benchmark Survey show that a 60 to 90 day payment cycle is the most common, followed by 45 to 60 days, for both large sites (those conducting 11 or more trials per year) and small sites (those conducting 10 or fewer trials). This remains largely unchanged from 2016 survey results...
Also in this issue:
- Regulatory Update
- FDA Actions