August 2015 – The CenterWatch Monthly : PDF
Product Details
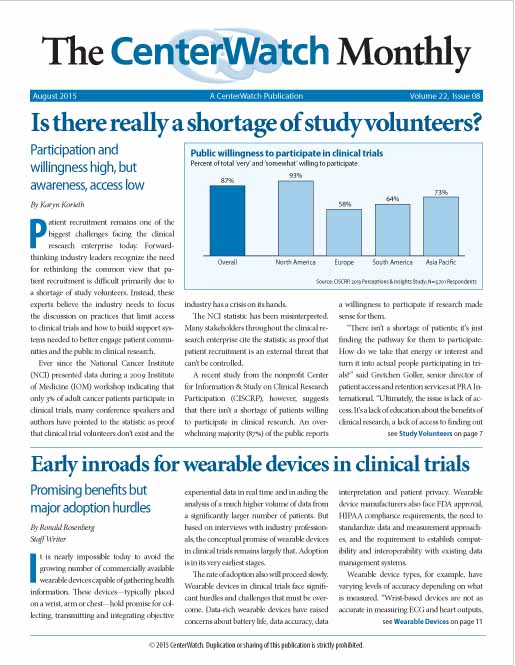
Is there really a shortage of study volunteers?
Patient recruitment remains one of the biggest challenges facing the clinical research enterprise today. Forward- thinking industry leaders recognize the need for rethinking the common view that patient recruitment is difficult primarily due to a shortage of study volunteers. Instead, these experts believe the industry needs to focus the discussion on practices that limit access to clinical trials and how to build support systems needed to better engage patient communities and the public in clinical research.
Early inroads for wearable devices in clinical trials
It is nearly impossible today to avoid the growing number of commercially available wearable devices capable of gathering health information. These devices—typically placed on a wrist, arm or chest—hold promise for collecting, transmitting and integrating objective experiential data in real time and in aiding the analysis of a much higher volume of data from a significantly larger number of patients. But based on interviews with industry professionals, the conceptual promise of wearable devices in clinical trials remains largely that. Adoption is in its very earliest stages.
Also in this issue:
- QT assessment has a heart-pounding new development
- Biosimulation takes center stage in determining pediatric dosing during clinical trial process
- Regulatory Update
- Month in Review
- FDA Actions
- Study Lead Opportunities
- New Drugs in the Pipeline