February 2011 – The CenterWatch Monthly : PDF
Product Details
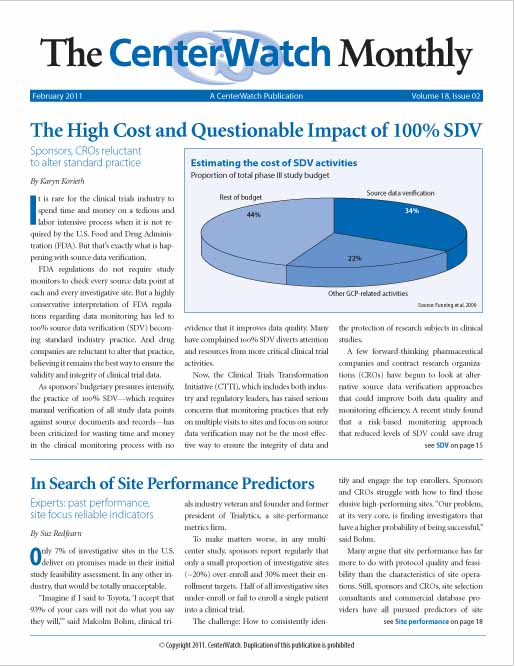
The High Cost and Questionable Impact of 100% SDV
It is rare for the clinical trials industry to spend time and money on a tedious and labor intensive process when it is not required by the U.S. Food and Drug Administration (FDA). But that’s exactly what is happening with source data verification.
In Search of Site Performance Predictors
Only 7% of investigative sites in the U.S. deliver on promises made in their initial study feasibility assessment. In any other industry, that would be totally unacceptable.
Eye On Genzyme
Genzyme, considered one of the world’s leading biotechnology companies, states its mission as offering major therapeutic advances to patients affected with serious diseases. Found in 1981, Genzyme currently employs 10,000 worldwide and generated $4.5 billion in revenues in 2009, its success attributed at least in part to its development and application of innovative life science technologies.
Also in this issue:
- How CROs can weather the consolidation storm
- Overcoming barriers to minority recruitment
- Industry Briefs
- The Pulse on Recruitment
- TrialWatch
- New Study Launches