August 2010 – The CenterWatch Monthly : PDF
Product Details
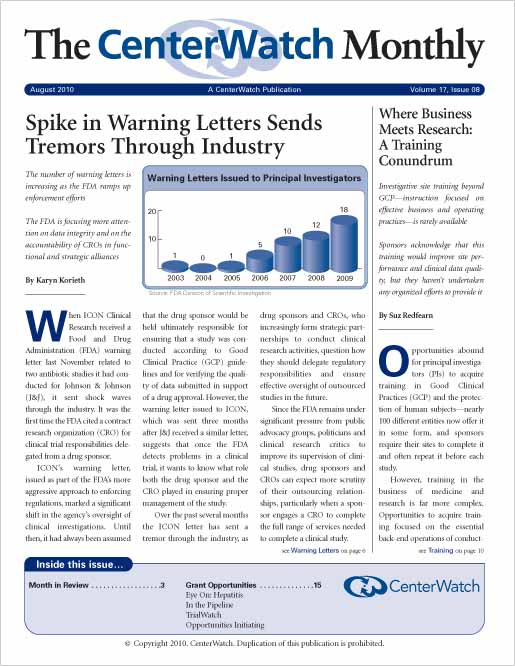
Spike in Warning Letters Sends Tremors Through Industry
The number of warning letters is increasing as the FDA ramps up enforcement efforts. The FDA is focusing more attention on data integrity and on the accountability of CROs in functional and strategic alliances. When ICON Clinical Research received a Food and Drug Administration (FDA) warning letter last November related to two antibiotic studies it had conducted for Johnson & Johnson (J&J), it sent shock waves through the industry. It was the first time the FDA cited a contract research organization (CRO) for clinical trial responsibilities delegated from a drug sponsor.
Where Business Meets Research: A Training Conundrum
Investigative site training beyond GCP—instruction focused on effective business and operating practices—is rarely available. Sponsors acknowledge that this training would improve site performance and clinical data quality, but they haven’t undertaken any organized efforts to provide it.
Eye On: Hepatitis
Infection with Hepatitis C virus (HCV) is the leading cause of chronic liver disease, with worldwide prevalence now exceeding 170 million, according to the World Health Organization. Transmission occurs through direct contact with infected blood, and complications may include cirrhosis, liver cancer (hepatocellular carcinoma; HCC) and death.Hepatitis B virus (HBV) may be transmitted through breaks in the skin, through sexual contact, and to infants born of infected mothers. Estimated worldwide prevalence of HBV infection is 2 billion, and of chronic HBV infection is 350 to 400 million, causing one million deaths annually from cirrhosis, liver failure, and HCC. Since an effective vaccine for HBV became available in the mid-1980s, incidence of acute HBV infection has markedly dropped in Western countries.
- Month in Review
- In the Pipeline
- TrialWatch
- Opportunities Initiating