November 2017 – The CenterWatch Monthly : PDF
$79.00
Product Details
Rethinking the Investigator’s brochure
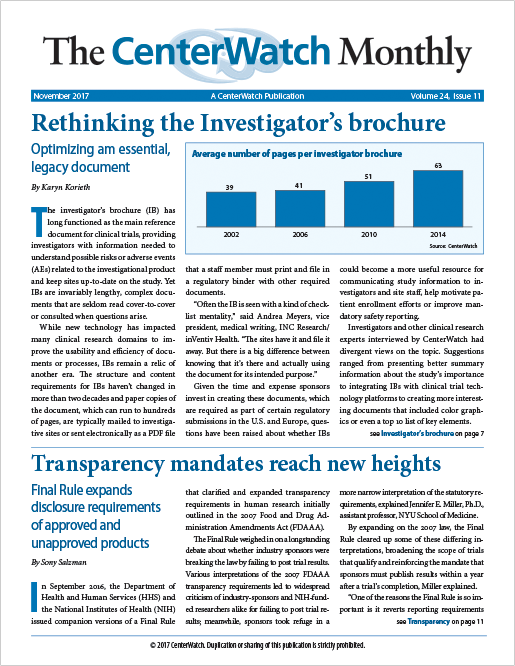
The investigator’s brochure (IB) has long functioned as the main reference document for clinical trials, providing investigators with information needed to understand possible risks or adverse events (AEs) related to the investigational product and keep sites up-to-date on the study. Yet IBs are invariably lengthy, complex documents that are seldom read cover-to-cover or consulted when questions arise.
Transparency mandates reach new heights
In September 2016, the Department of Health and Human Services (HHS) and the National Institutes of Health (NIH) issued companion versions of a Final Rule that clarified and expanded transparency requirements in human research initially outlined in the 2007 Food and Drug Administration Amendments Act (FDAAA).
Also in this issue:
- The Gold Rush and Monopoly Land grab in eClinical growth
- Increasing participation in clinical trials among underrepresented populations
- Pay clinical trial patients back with lay language summaries
- Regulatory Update
- Month in Review
- FDA Actions
- Study Lead Opportunities
- New Drugs in the Pipeline