October 2013 – The CenterWatch Monthly : Print
Product Details
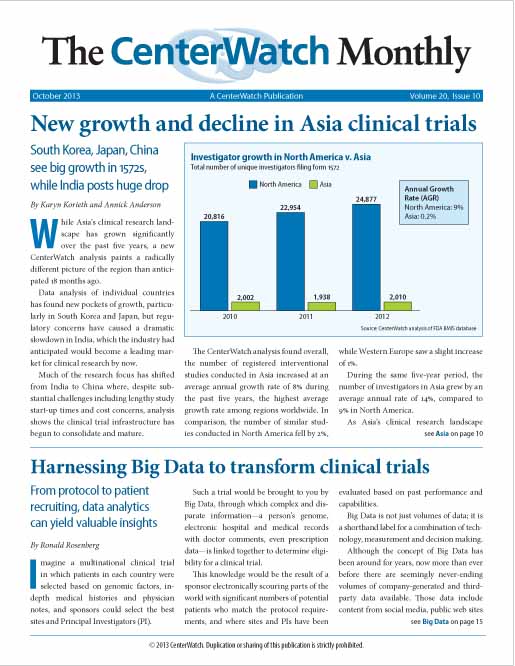
New growth and decline in Asia clinical trials
While Asia’s clinical research landscape has grown significantly over the past five years, a new CenterWatch analysis paints a radically different picture of the region than anticipated 18 months ago. Data analysis of individual countries has found new pockets of growth, particularly in South Korea and Japan, but regulatory concerns have caused a dramatic slowdown in India, which the industry had anticipated would become a leading market for clinical research by now.
Harnessing Big Data to transform clinical trials
Imagine a multinational clinical trial in which patients in each country were selected based on genomic factors, indepth medical histories and physician notes, and sponsors could select the best sites and Principal Investigators (PI). Such a trial would be brought to you by Big Data, through which complex and disparate information—a person’s genome, electronic hospital and medical records with doctor comments, even prescription data—is linked together to determine eligibility for a clinical trial.
Also in this issue:
- ACPU aims to improve phase I safety, quality
- SMARTMonitoring™ supports sites and sponsors
- Regulatory Update
- Month in Review
- FDA Actions
- Study Lead Opportunities
- New Drugs in the Pipeline