December 2011 – The CenterWatch Monthly : PDF
Product Details
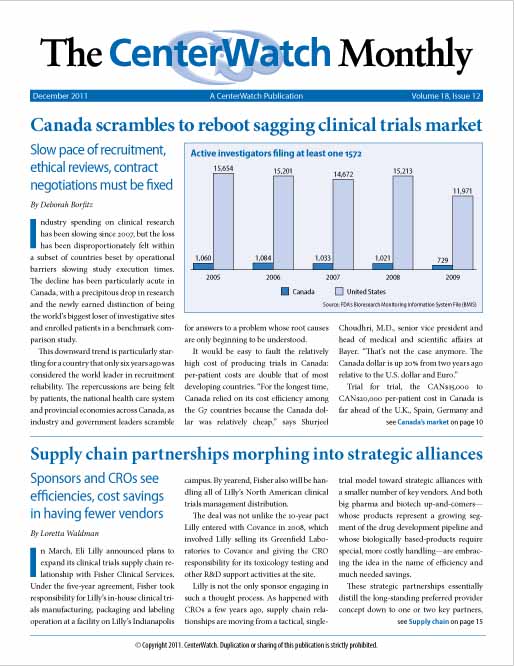
Canada scrambles to reboot sagging clinical trials market
Canada has seen a precipitous drop in research and has the newly earned distinction of being the world’s biggest loser of investigative sites and enrolled patients in a benchmark comparison study, a downward trend particularly startling for a country that only six years ago was considered the world leader in recruitment reliability. A confluence of factors has made Canada a slow and expensive location for trials, and rebooting the market will require...
Supply chain partnerships morphing into strategic alliances
As happened with CROs a few years ago, supply chain relationships are moving from a tactical, single-trial model toward strategic alliances with a smaller number of key vendors. And both big pharma and biotech are embracing the idea in the name of efficiency and...
Eye On Eli Lilly
Eli Lilly has a history of innovation beginning in 1876 and is now the tenth largest pharmaceutical company worldwide. Its global presence includes clinical research performed in more than 50 countries. Lilly’s R&D focus is to generate potential biotech solutions for a wide range of diseases, and to pursue personalized medicines...
- CRAs must form positive relationships
- Increasing the effectiveness of novel imaging in trials
- Regulatory Update
- Month in Review
- CRO Industry Update
- TrialWatch
- New Study Launches