August 2011 – The CenterWatch Monthly : PDF
Product Details
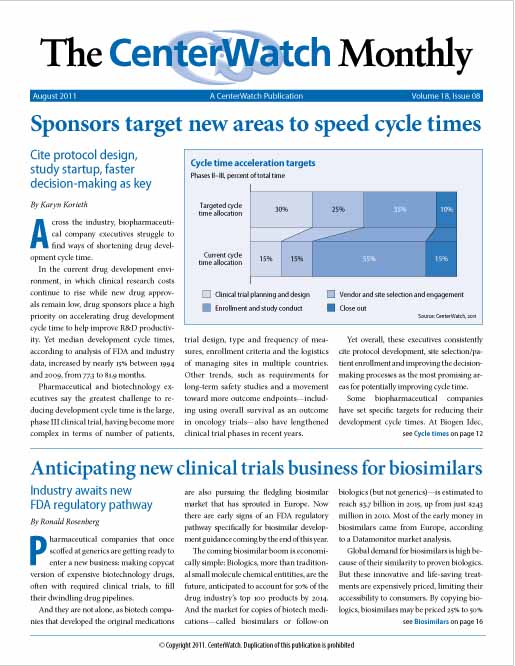
Sponsors target new areas to speed cycle times
Biopharmaceutical company executives struggle to find ways of shortening drug development cycle time. Sponsors place a high priority on accelerating cycle time to help improve R&D productivity, yet median cycle times, according to analysis of FDA and industry data, increased by nearly 15% between 1994 and 2009, from 77.3 to 81.9 months. Executives say the greatest challenge to reducing cycle time is the large, phase III clinical trial, which has become more complex. Yet overall, these executives say the most promising areas for improving cycle times are...
Anticipating new clinical trials business for biosimilars
Pharmaceutical companies that once scoffed at generics are getting ready to enter a new business: making copycat version of expensive biotechnology drugs, often with required clinical trials, to fill their dwindling drug pipelines. And they are not alone, as biotech companies are also pursuing the fledgling biosimilar market that has sprouted in Europe. Now there are early signs of an FDA regulatory pathway specifically for biosimilar development...
Eye On Novo Nordisk
Novo Nordisk, a global healthcare company headquartered in Denmark, opened in 1923. In addition to pursuing pharmacotherapy for diabetes and other Endocrinology indications, Novo Nordisk is in active development of drug candidates in Hematology and Inflammation...
- The impact of imaging on drug development programs
- Controlling monitoring costs
- Industry Briefs
- The Pulse on Recruitment
- TrialWatch
- New Study Launches