March 2011 – The CenterWatch Monthly : PDF
Product Details
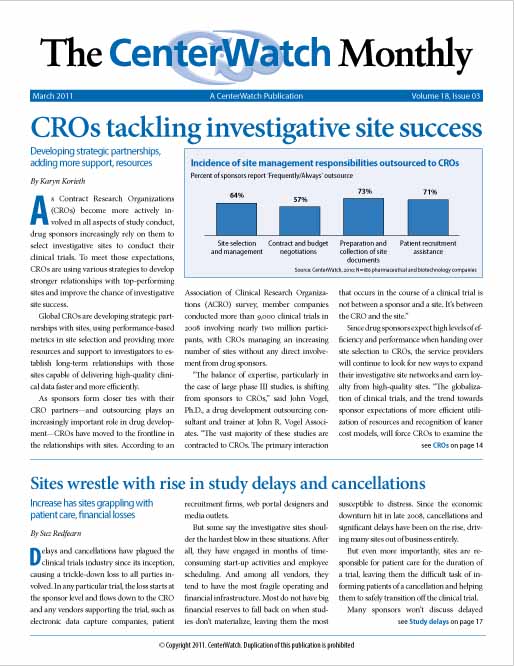
CROs tackling investigative site success
As Contract Research Organizations (CROs) become more actively involved in all aspects of study conduct, drug sponsors increasingly rely on them to select investigative sites to conduct their clinical trials. To meet those expectations, CROs are using various strategies to develop stronger relationships with top-performing sites and improve the chance of investigative site success.
Sites wrestle with rise in study delays and cancellations
Delays and cancellations have plagued the clinical trials industry since its inception, causing a trickle-down loss to all parties involved. In any particular trial, the loss starts at the sponsor level and flows down to the CRO and any vendors supporting the trial, such as electronic data capture companies, patient recruitment firms, web portal designers and media outlets.
Eye On Amgen
Amgen is a pioneering biotechnology company in human therapeutics, with more than 25 years of experience applying scientific breakthroughs to the development of new biologic agents. Utilizing advances in recombinant DNA, cellular and molecular biology, Amgen developed the first new products in this field to improve health in millions of people with cancer, renal disease, rheumatoid arthritis, bone
Also in this issue:
- Industry Briefs
- The Pulse on Recruitment
- TrialWatch
- New Study Launches