October 1999 – The CenterWatch Monthly : PDF
$79.00
Product Details
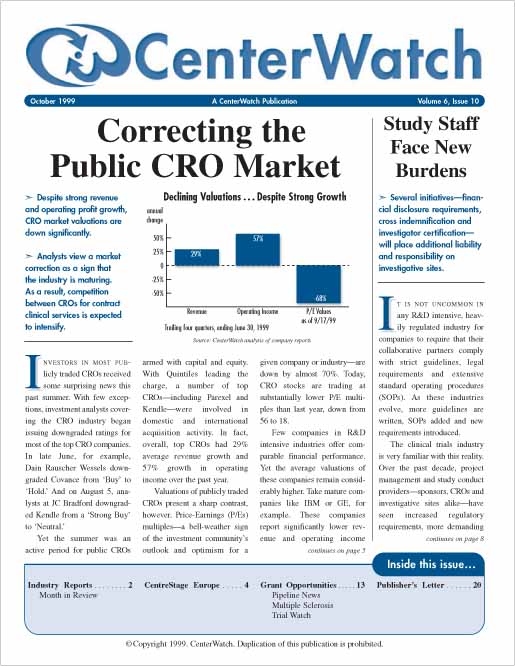
Correcting the Public CRO Market
Despite strong revenue and operating profit grown, CRO market valuations are down significantly.
Analysts view a market correction as a sign that the industry is maturing. As a result, competition between CROs for contract clinical services is expected to intensify.
Study Staff Face New Beasts of Burden
CenterWatch editorial examines several initiatives -- Financial disclosure requirements, cross indemnification and investigator certification -- that threaten to place additional liability and responsibility pressure on investigative sites.
Also in this issue:
- CentreStage Europe: A Look at SMO usage in Continental Europe
- Eye On: Multiple Sclerosis