
Home » Protocol Amendments Continue to Increase, Report Shows
Protocol Amendments Continue to Increase, Report Shows
March 6, 2023

Despite sites calling for a reduction in the amount of burdensome protocol amendments from sponsors, a newly published study shows that the prevalence and average number of amendments are still on the rise.
Out of 952 total protocols assessed by the Tufts Center for the Study of Drug Development (CSDD) between June and October 2022, three-quarters had at least one substantial amendment, defined as protocol changes requiring both internal/external approval and reconsenting of participants.
Just over half of protocols (57 percent) had amendments in 2015, CSDD reports.
“Protocol amendments play a major role in driving up clinical trial costs and timelines. Their impact is not only highly disruptive, it is unplanned and unbudgeted,” Ken Getz, executive director of CSDD, told CenterWatch Weekly. “The prevalence and frequency of amendments is rising in step with increasing protocol complexity.”
The prevalence of substantial amendments is remarkably high in the current landscape but varies by trial phase, CSDD found, with nearly nine in 10 phase 2 trials seeing at least one (89 percent), followed by phase 3 (82.4 percent), phase 1 (67 percent) and postmarket trials (54.2 percent).
The CSDD’s latest Impact Report also shows that clinical teams implement an average of 3.3 amendments of any type (substantial and insubstantial), with the highest average number seen in phase 3 trials (3.5 amendments), trailed by phase 2 (3.3), phase 1 (3.1) and postmarket trials (2.4).
CSDD researchers concluded amendments are associated with larger and more complex trials as well as higher numbers of sites/countries, participants screened/enrolled, endpoints and eligibility criteria.
Regulator requests and shifts in trial strategies were the most common reasons by far for implementing an amendment, compared to a 2010 CSDD study that identified new safety data as the primary cause.
In terms of the types of changes amendments entailed, CSDD found that nearly 72 percent required modifications to study assessments, 70.2 percent necessitated changes to trial designs, 65.1 percent changed patient selection, withdrawal and treatment parameters, and 65 percent made changes to general trial information.
Additionally, 56.1 percent and 43.3 percent of amendments required changes in trial objectives and statistical analysis plans, respectively. Just 13.2 percent involved operational changes, including modifications to supplemental materials, quality control measures, financial elements and insurance.
CSDD also looked at protocol amendments’ impact on trial performance.
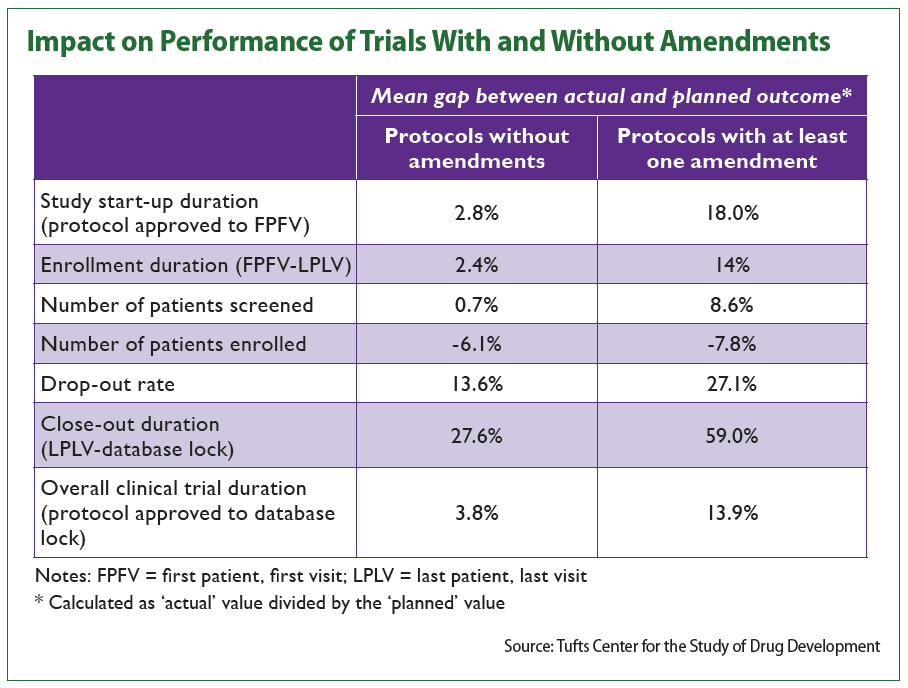
Study startup duration (from protocol approval to first patient, first visit), for example, saw an average gap between actual and planned outcomes of 2.8 percent in trials with no amendments and a gap of 18 percent in trials with at least one amendment.
Similarly, trial closeout durations (from last patient, last visit to database lock) saw a 59.1 percent gap between planned and actual durations in protocols with amendments compared to 27.6 percent in protocols without.
Additionally, CSDD found that protocols requiring at least one amendment generally screened a larger number of patients. On the other hand, however, these trials recruited fewer participants than planned, the data show.
Growing protocol complexity, which has been identified as a prominent trend in clinical research, especially in oncology, has been named a significant pain point by sites that they say they want sponsors to address.
Sandy Smith, WCG Clinical’s senior vice president of clinical solutions and strategic partnerships, recently reported that sites want sponsors to cut down on final protocol delivery times and the numbers of protocol amendments they need to contend with. The issue has come to the point where some sites refuse to proceed until the final protocol is done and ready, putting preliminary work on hold and adding to trial durations (CenterWatch Weekly, Feb. 27).
Access the CSDD Impact Report here: https://bit.ly/3ENKYru.

Upcoming Events
-
07May
-
14May
-
23May
-
21Oct




