
Home » CNS Trials Look to Decentralized Methods to Help Solve Enrollment Challenges
CNS Trials Look to Decentralized Methods to Help Solve Enrollment Challenges

March 7, 2022

Patient recruitment, not study startup, is the biggest obstacle in conducting central nervous system (CNS) trials, which increasingly use decentralized trial (DCT) methods to ease the way, a new survey shows.
Almost half (46 percent) of respondents to a recent survey by Science 37, a clinical research company specializing in DCTs, named recruitment as their single greatest challenge to running their trials.
The runner-up — endpoint collection — wasn’t even close, with just 22 percent of respondents naming it as their greatest challenge. Patient retention was named by 15 percent of the 72 respondents — executive-level corporate decisionmakers primarily in clinical operations and R&D at pharma/biotech and medical device companies, and CROs — while study startup and project management came in at 8 and 4 percent, respectively.
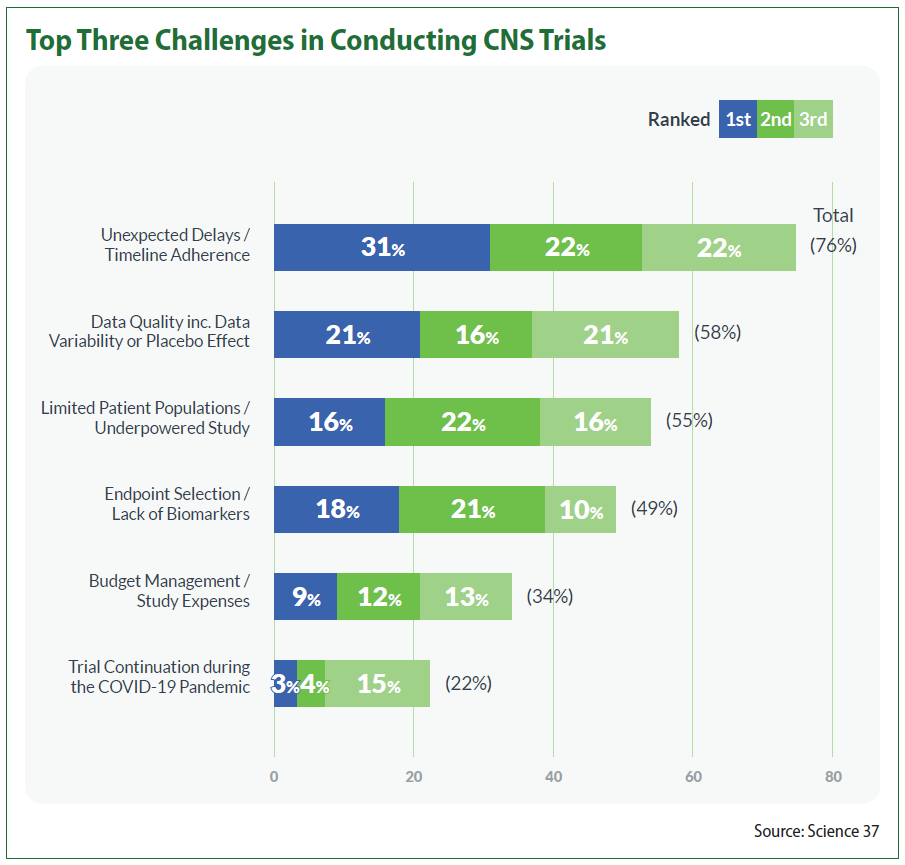
Survey respondents also were asked to rank the top three issues that keep them up at night when conducting CNS trials, with 76 percent choosing unexpected delays/timeline adherence and 58 percent placing data quality as it pertains to conducting the study (including data variability or placebo effect) in their top three. Similarly, limited patient populations/underpowered trials and endpoint selection/lack of biomarkers came in at 55 and 49 percent, respectively.
But the survey respondents indicated some DCT approaches, such as telehealth visits, mobile nursing services and electronic reporting, can help. Of the respondents, 56 percent placed greater patient retention at the top of their list of DCT benefits, followed closely by increased patient diversity (54 percent) and accelerated patient recruitment (52 percent). The ability of hybrid DCTs to expand trial participation to people who live in areas with little access to study sites is seen as a boon, especially for site staff, who frequently struggle to enroll trial participants and deal with patients who have to travel often and/or far to participate.
Sites taking part in CNS trials should be prepared for a significant shift; 67 percent of respondents said they intend to conduct either hybrid or fully remote DCTs in the next year, a notable increase from the 49 percent seen in the previous 12 months.
Sixty percent have their sights set on hybrid trials, up from 46 percent in the past year. By contrast, just 26 percent are confident enough at this point to plan fully decentralized trials, though this was a 12-percentage point increase. The scales appear to be tipping; 57 percent of respondents said they were planning to run traditional site-based trials in the next 12 months, a marked drop from the 74 percent seen in the prior year.
The survey found that 72 percent of respondents intend to incorporate electronic patient-reported outcomes (ePRO) and electronic clinical outcome assessment (eCOA) tools into trials in the next year, while 56 percent said they intend to employ mobile nurses, eConsent and telemedicine in their trials in the next 12 months. Additionally, 48 percent plan to bring wearables/sensors into their trials, while 44 percent said they would establish remote sites and 36 percent said they would involve local clinics in their trials.
Mobile nurses in particular can help to improve patient recruitment and retention in CNS trials, especially with regard to certain diseases, according to Chris Reist, medical director of psychiatry and behavioral sciences for Science 37. The company anticipates a 40 percent increase in the use of mobile/home nurses in CNS trials in the next year.
“When you get outside of a 20- or 25-mile radius from a site, you really start having dropout in terms of people just not willing to make that trek. I think that totally changes when we come to the patient,” he said. “With Alzheimer’s, Parkinson’s, often mobility is an issue, and you’re not only dealing with the patient’s schedule, you’re having to accommodate the caregiver’s schedule.”
The survey showed that Alzheimer’s disease topped the list of CNS conditions that executives planned to use DCT approaches for, with 35 percent of respondents saying they intended to do so. Major depressive disorder, Parkinson’s and multiple sclerosis all tied at 26 percent, while other conditions, such as post-traumatic stress disorder (PTSD), seizures/epilepsy and schizophrenia were cited to a lesser degree.
Some CNS indications are better suited for certain DCT approaches than others, according to David Kudrow, medical director of neurology for Science 37. Migraine and headache disorders more broadly “are perfectly conducive to DCTs,” as data in those trials are primarily quality-of-life data, PROs and electronic headache diaries that determine most primary and secondary outcome measures, Kudrow said. Similarly, epilepsy trials use the collection of seizure records/diaries and industry is moving toward greater use of electronic diaries in these trials, he said.
But even if a trial doesn’t seem perfectly suited for complete decentralization, certain DCT methods may still be useful, Reist said. “One might presume if a trial involves … for example, specialized imaging, that might be a deal breaker, but that’s where you bring in the [hybrid] concept,” he said. “You can have a lot of the components that are done remotely and then utilize local services for those unique kinds of tests or procedures.”
Fifty-one percent of respondents said they had done a DCT viability assessment for at least one CNS trial in the past year, while 49 percent said they had not considered the question. Of those that assessed viability, 36 percent decided to proceed with using DCT components while 15 percent opted not to. Those that did choose to move forward cited a number of reasons, chief among them increased diversity, improved retention, better patient experience and faster recruitment.
Those that chose not to use DCT components were strongly swayed by a need for human interaction at sites, concerns over data quality, complexities in their trial requirements and the need to integrate DCT elements at sites, among other factors.
The shift toward DCTs “is undeniable,” says Robert Cowan, professor of neurology and chief of the division of headache medicine at Stanford University. But because decentralization can be a double-edged sword, its impact on sites and trials, good or bad, will depend on implementation.
For instance, while it can be used to increase the diversity of trials and identify the best potential participants, it can also lessen the input that specialized experts at sites have during a trial, he said. At the same time, it can be an invaluable way to significantly cut costs and accelerate recruitment time and, “perhaps most importantly, improve comparability and consistency among and within … sites.”
Nathaniel Katz, chief science officer of WCG Analgesic Solutions, recommends that sites build relationships with organizations that send research professionals to participants’ homes in case a sponsor requires that capability. In addition, having systems established to handle the logistics of transportation, communication, meals and other areas that allow participants to choose between remote or in-person is still a highly valuable site asset in the eyes of sponsors, he said. But most importantly, the most attractive site quality is, as always, access to patients. Sites with access to large databases of qualified patients “will always be the most precious commodity in the clinical trials space,” Katz told CenterWatch Weekly.
One of the biggest hurdles for sites appears to be all the technology and training thrown at them by DCT approaches, according to Noolie Gregory, vice president of operations management, decentralized solutions at Syneos Health.
“As an industry, we need to better streamline so sites do not fall victim to every protocol with its own technological approach to manage,” she said.
Read the full report here: https://bit.ly/3HIIj0K.


Upcoming Events
-
23Apr
-
07May
-
14May




