
Home » COVID-19 Trial Disruptions Total More Than 1,100, Study Shows
COVID-19 Trial Disruptions Total More Than 1,100, Study Shows

May 18, 2020

COVID-19 has caused nearly half of all trial disruptions in the past five months, with oncology trials being hardest-hit, according to a new study out of Berlin.
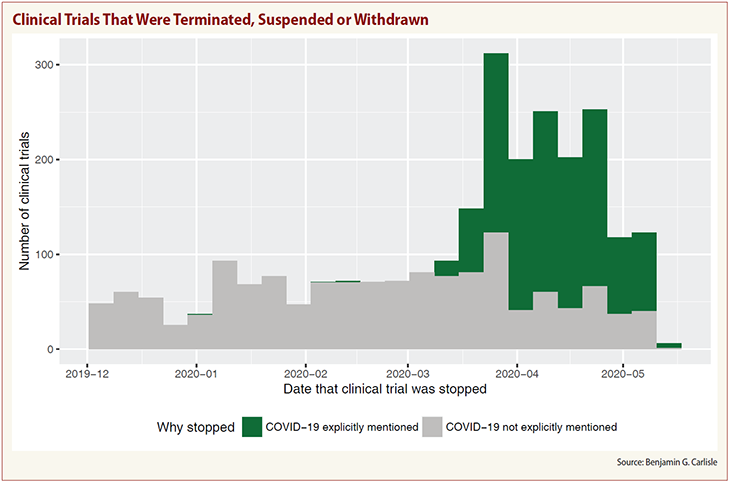
Forty-four percent of the more than 2,600 clinical trials stopped in the past five months for any reason have cited the COVID-19 outbreak as the cause, the ongoing study shows.
More than 1,100 trials have been impacted by COVID-19 since December with 97 percent suspended, 2 percent terminated and 1 percent withdrawn, according to a study of 90,000 trials that seeks to analyze the effect of the pandemic on sponsors, research sites and patients.
Of all therapeutic areas, oncology trials are bearing the brunt of the crisis with 28 percent of studies affected, followed by cardiovascular at 11 percent, neurologic at 8.5 percent and pain management at 5.2 percent, the study from Benjamin Carlisle of university hospital Charité in Berlin shows.
Trials disrupted by COVID-19 involve nearly 40,000 already-enrolled patients and 4 million more planned enrollees. Most of the trials — 84 percent — are interventional and 15 percent are observational.
Reasons listed for suspending trials include such statements as “Healthy volunteer enrollment on hold at site due to COVID-19 pandemic,” “Coronavirus stay-at-home orders prevent in-person data collection” and “Study halted prematurely due to COVID-19 but potentially will resume. Follow-up visits continue virtually.”
Studies terminated/withdrawn cite such reasons as loss of funding, disruption of research team, cessation of nonessential clinical activities, recruiting problems and small numbers of new participants enrolled.
While most sponsors and sites intend to revive their trials, says Carlisle, the future doesn’t look promising. “The prospect of starting again is far from certain for any clinical trial that has stopped, and even in cases where a clinical trial resumes after the pandemic, there may be reduced statistical power, more funding needed or changes to the protocol to accommodate for the interruption,” Carlisle says.
Carlisle is gathering data from trials listed on ClinicalTrials.gov to assess the nature of COVID-19 disruptions and hopefully provide some insight into when recovery could occur. Examining data on the nearly 1,300 trials suspended in the same five-month period two years ago, he found only 9.5 percent of them were restarted within one year.
Carlisle is updating the numbers daily as the pandemic continues. He plans to continue collecting data until there has been a two-week period without a COVID-19 trial disruption or on Nov. 30, whichever comes first.
To view study data and the current report, click here: https://bit.ly/2WWus1F.
Related Directories
Upcoming Events
-
07May
-
14May




