
Home » Traditional vs. Pragmatic: Changing the Trial Model with Real-World Evidence
Traditional vs. Pragmatic: Changing the Trial Model with Real-World Evidence

January 24, 2020
Studies based on real-world evidence (RWE) don’t have to be a replacement for randomized clinical trials but rather an integral part of an overall trial strategy.
Both pragmatic and randomized trials have their pros and cons, but when combined they can produce more effective results. “I think using both of these to provide evidence to the regulators will be where we go in the future,” said Francis Kendall, senior director at Cytel.
You could conduct one or two small phase 1 or 2 trials to show safety and efficacy, he suggested, then apply machine learning and analytics to data on the full targeted population to confirm results. “And if things are not going quite to plan, that’s when you bring in a controlled trial” as backup.
The pragmatic approach is especially beneficial with hard-to-reach populations and studies of rare diseases with a limited number of patients. Registry data and electronic health records can provide evidence where a randomized clinical trial might not be possible.
Other benefits of using data generated in pragmatic trials include:
- Using predictive analytics to identify candidates for clinical trials;
- Getting a closer and earlier look at the “average patient;”
- Developing biomarkers to support outcomes;
- Optimizing protocols; and
- Targeting patients and physicians for recruitment.
“Everybody’s starting to use information in different ways, and we have to think about how the clinical development process is actually changing to adapt to these different sources of data,” Kendall said.
Kendall also said that as the population ages, it will be more important to understand patients’ development and journey through healthcare. “We can’t just do clinical trials. We need real-world evidence to have a look at that,” he added.
“Data science in pharma is more than clinical development, is more than just looking at evidence for efficacy … and then safety,” Kendall said. “It’s about targeting patients, understanding patients’ journeys and really helping the patient, putting the patient at the center.”
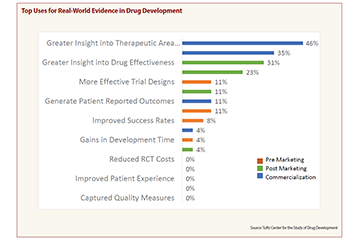
A survey conducted by the Tufts Center for the Study of Drug Development shows sponsors value RWE the most for the insights it provides into therapeutic area needs, market access and drug effectiveness (CenterWatch Weekly, Aug. 26, 2019). Survey respondents also said they use RWE to improve trial design, improve the safety of marketed drugs and gain a faster regulatory response to their applications.
“I can see in the future, the regulators will want to see … evidence of not only the clinical trial, but maybe a pragmatic clinical trial, or at least a trial done in different settings of standard of care,” Kendall predicted. But drug developers have not yet fully taken the plunge. “We can see some evidence of pharma interest in the mix, but they’re still dipping their toe in the water.”
Upcoming Events
-
23Apr
-
07May
-
14May




