
Home » Centralized Monitoring in Practice — Lessons Learned from Early Implementers
Centralized Monitoring in Practice — Lessons Learned from Early Implementers
January 21, 2019
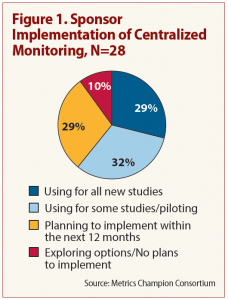
A survey conducted recently by Metrics Champion Consortium shows most of the responding sponsors — 90 percent — indicate they are implementing ICH’s new centralized monitoring approach to risk management, but only about a third of that number say they have fully integrated it into their trials operations. See Figure 1.
Almost all (93 percent) cited detecting risks to subjects and data integrity as the primary reason to conduct centralized monitoring, and three quarters of respondents said it was both a good predictor of the need for individual site visits and detector of potential fraud and/or misconduct among trial staff.
“Onsite monitors are not able to see how their sites compare to others in the same study — whether their performance, data trends, etc., are outliers compared to other sites,” says MCC Executive Director Linda Sullivan. A particular benefit of centralized monitoring is the ability to identify whether data is being fabricated, Sullivan says, something very difficult for an onsite monitor to do. See Figure 2.
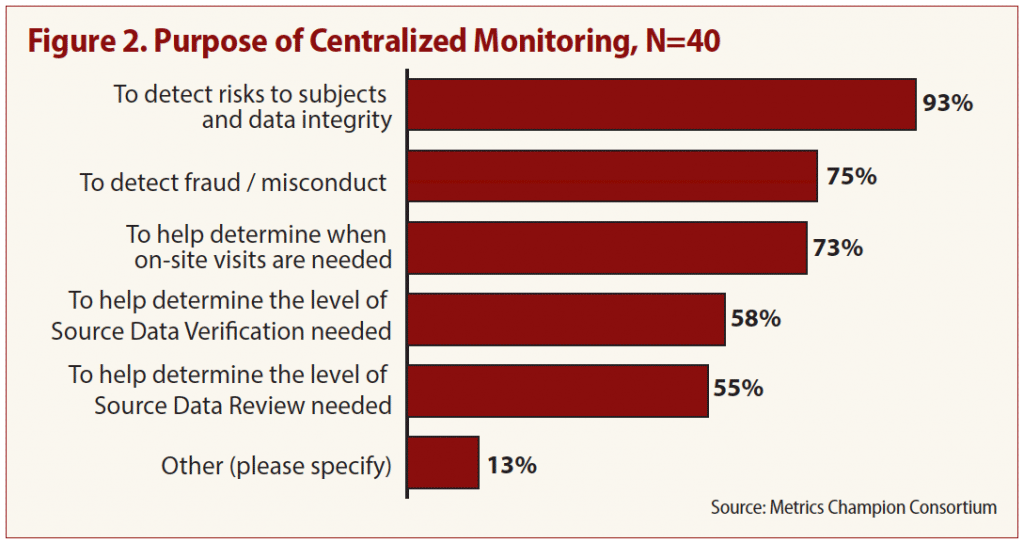
It’s surprising, however, that so few respondents (58 percent) named source data verification (SDV) assessment as a primary purpose, says study coauthor Keith Dorricott of Dorricott Metrics and Process Improvement. One of the driving factors of the move to risk-based monitoring, he says, was recognition that using SDV alone to spot problems was unreliable.
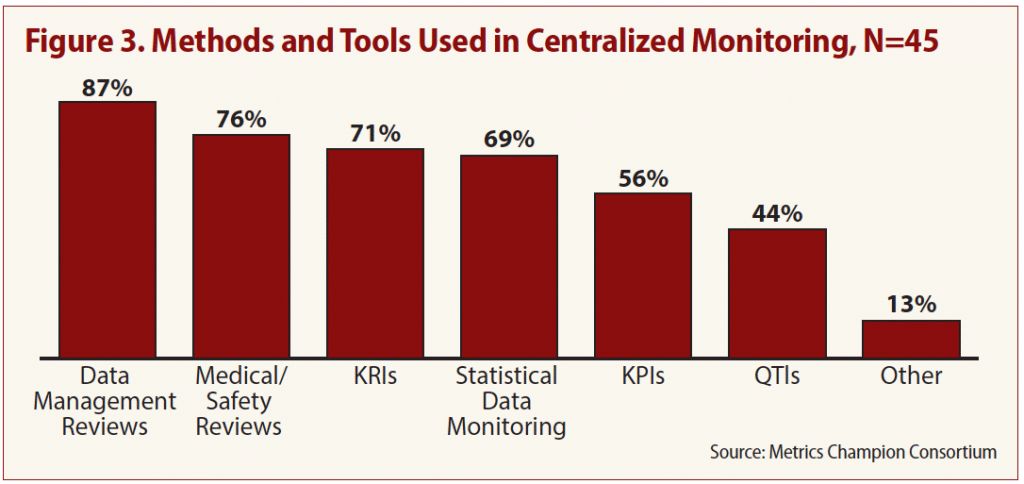
Tools and methods respondents commonly use in centralized monitoring, according to the survey, include:
- Data management reviews (87 percent);
- Medical/safety reviews (76 percent);
- Statistical data monitoring (SDM) (69 percent);
- Setting key risk indicators (KRI) (71 percent); and
- Identifying key performance indicators (KPI) (56 percent).
But one of the most effective methods, setting quality tolerance limits (QTL), is used by fewer than half (44 percent) of respondents (see Figure 3).
ICH E6 (R2) recommends setting predefined QTLs, “taking into consideration the medical and statistical characteristics of the variables as well as the statistical design of the trial.” Deviations from established QTLs, the guideline says, “should trigger an evaluation to determine if action is needed.”
Survey respondents seem to have recognized the value of using QTLs, however; almost half of those not currently employing QTLs in centralized monitoring say they plan to add the tool in the coming year.
Survey responses also indicate a mixed approach to organizational implementation of centralized monitoring. Most respondents (57 percent) disperse responsibilities across existing departments, compared to 38 percent that house the function in its own central group.
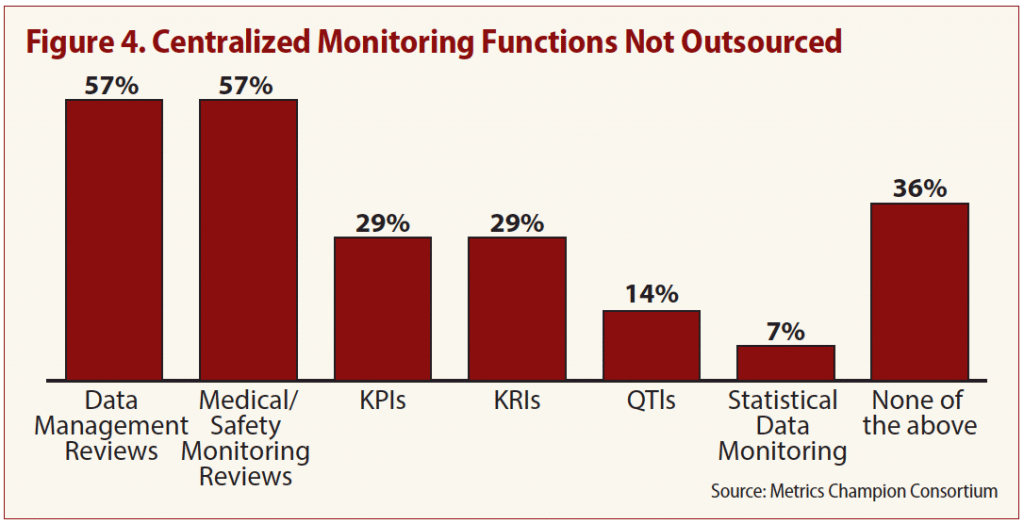
Only 5 percent of respondents said they outsource such centralized monitoring functions as KPIs, KRIs, QTLs and statistical data monitoring. But the majority of those that outsource indicated there are two vital functions they don’t let out of their hands: data management review and medical/safety monitoring reviews (see Figure 4).
Upcoming Events
-
23Apr
-
07May
-
14May




